U32nrt
They have completely omitted the gas constant.

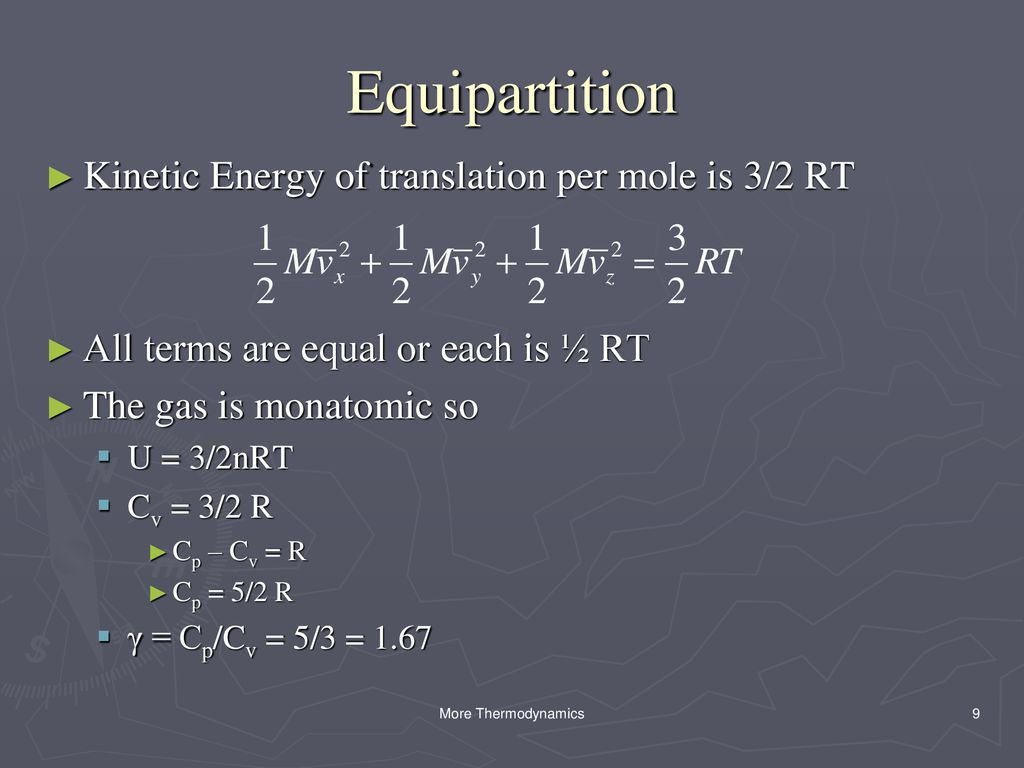
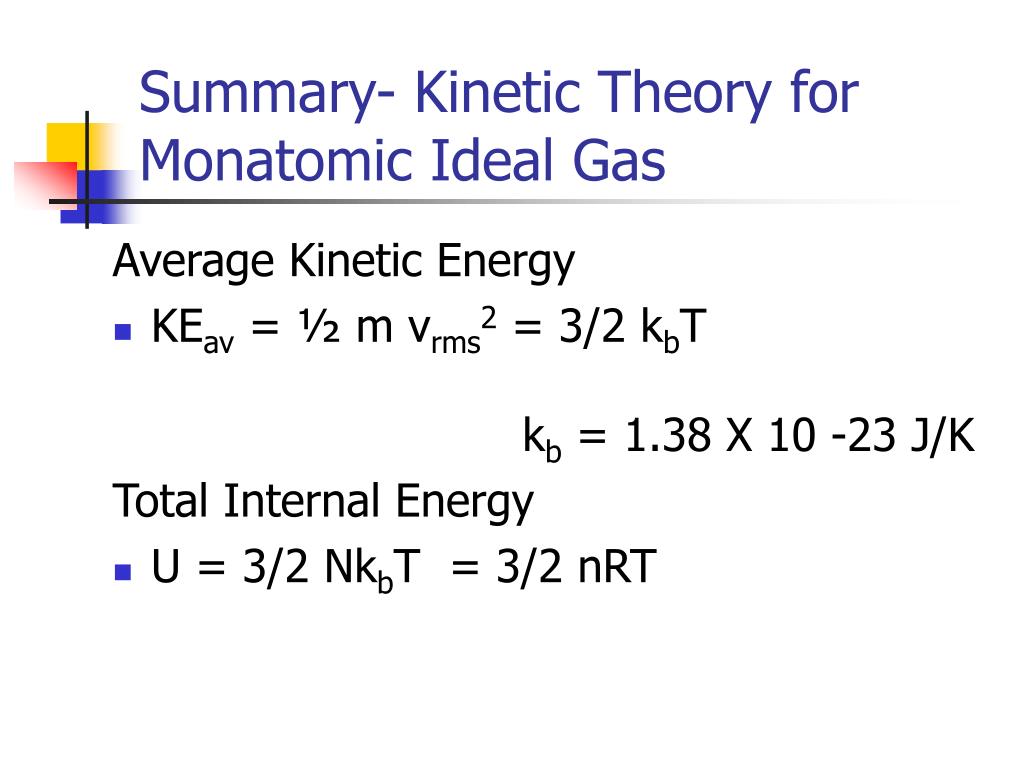
U32nrt. E' solo energia cinetica:. Science defines the systematic means by which we obtain knowledge and gain a better understanding of the universe. KT= 2 3 U N = 2 3 hKi () The temperature of an ideal gas is a measure of the average kinetic energy of the constituents.
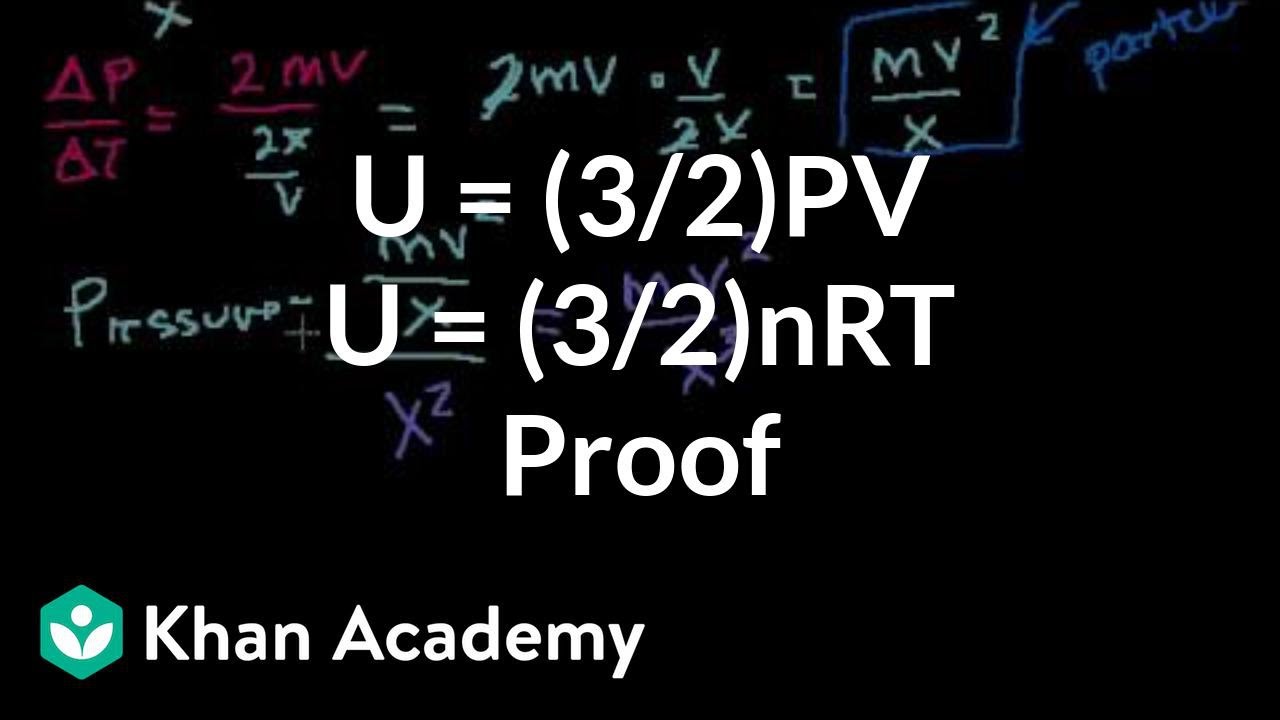
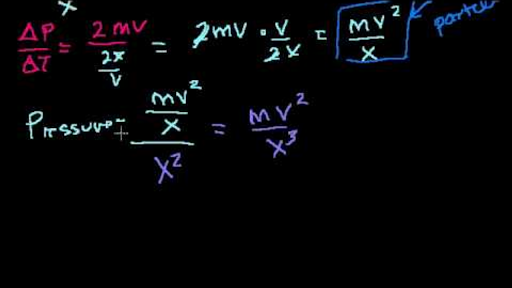
My thinking was this:. The box contains N molecules of ideal gas, each of mass m. The simple answer is that the 2 comes from the equation for kinetic energy, 1/2mv^2 which you should have had in physical science.
S (or Entropy) is a valid state variable;. Reviews There are no reviews yet. Consider a cubical box, L on each side.
Topics Salman Khan, Khan Academy. Reconciling thermodynamic and state definitions of entropy. U = (3/2)PV or U = (3/2)nRT.
Why does U = (3/2)nRT?. This is the currently selected item. 1) According To The Kinetic Theory Of Gases, The Translational Kinetic Energy Of A Monatomic Ideal Gas Is U= (3/2)nRT Where N Is The Number Of Moles Of Gas.
This thread is archived. Conceptual proof that the internal energy of an ideal gas system is 3/2 PV. Thermal Energy sum of the total random kinetic energy that particales have U=3/2nRT 3 7.
TAGS Thermodynamics, Energy, Heat, NRG. U = (3/2)pv или u = (3/2)nrt Това е елементът, който е избран към настоящия момент. 0°C = 273.15 K 1L = 1 dm.
Conceptual proof that the internal energy of an ideal gas system is 3/2 PV. It states that we add 1/2KT per degree of freedom and 1KT per degree of Vibrational freedom. Than around 150 K:.
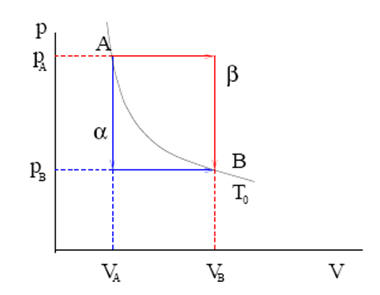
Are there any example questions that utilize one vs the other to show. For a van der Waals gas, the pressure P and the internal energy U, as a functions of n, V, and T are given by P=nRT/V-nb -n^2a/V^2 and. U=(3/2)PV or U=(3/2)nRT Video is suitable for 7th - 10th Grade.
For A Diatomic Gas. Absolute zeroThe lowest temperature that is theoretically possible. For A Monatomic Gas.
You've reached the end of your free preview. Never gonna give you up. Trabalho feito por processo isotérmico.
<K> = (3/2) kT (4.5) The root-mean-square of speed v rms is defined as:. Carnot Cycle and Carnot Engine;. Because the internal energy of an ideal gas is U = (3/2)nRT, the work done is the following:.
Sal goes into how to mathematically measure the amount of internal energy that is present in a system;. It gets a bit complicated to derive and you have to go through a long process. Energia interna di un gas perfetto:.
Work done by isothermic process. This is done by the equi partition theorem. Watch the next lesson:.
Работа, извършена при изотермен процес. C(ethanol) = 2.42 J g K⋅⋅ − −11. Work Done by Isothermic Process;.
Hot Products Antigens & Antibodies for Diagnostic Kit DevelopmentNew SARS-CoV-2 Related ProductsNew CD19 - a validated therapeutic target for B-cell malignanciesNew CD3 Protein - A hot target for bispecific antibodyHot CD24 & Siglec-10 Proteins - Hot Targets for Tumor ImmunotherapyNew CD73 - A novel target for immunotherapyNew Siglec-15 - Validated by Cell-based Assay BLI/ SPR/ ELISANew CD. Reconciling thermodynamic and state definitions of entropy. U = (3/2)nRT = (3/2) NkT (4.4) Where N A = 1.023 x 10 23 the Avogadro's number and k = R/N A = 1.38 x 10 - 23 T/K the Boltzmann Constant.
PAd = some tiny work done by n moles of the gas, d is a tiny distance moved v = Ad = tiny volume expanded or contracted N/n = ratio between total moles. My lecturer said it had to do with some quantum mechanics, but I never really got any answers from him. Thermodynamic entropy definition clarification.
A) Compute AU And AH When 2.0 Mol Of Gas Increases In Temperature From 300 To 600 K. For temperatures above 150 energy can be transfered into rotating the molecules, and the change in energy U= (5=2)nR T. 1 nm = 10-9.
Moderator of r/dankmemes, speaking officially Score hidden · 2 years ago · Stickied comment. Thermodynamic entropy definition clarification. We can calculate enthalpy change this way for any system because enthalpy is a state function.
Volume Ratios in a Carnot Cycle;. Am I right in thinking that 3/2nRT is used for calculating total KE of a system, whereas 3/2RT is avg kinetic energy?. Explore this incredibly broad discipline with the following online science.
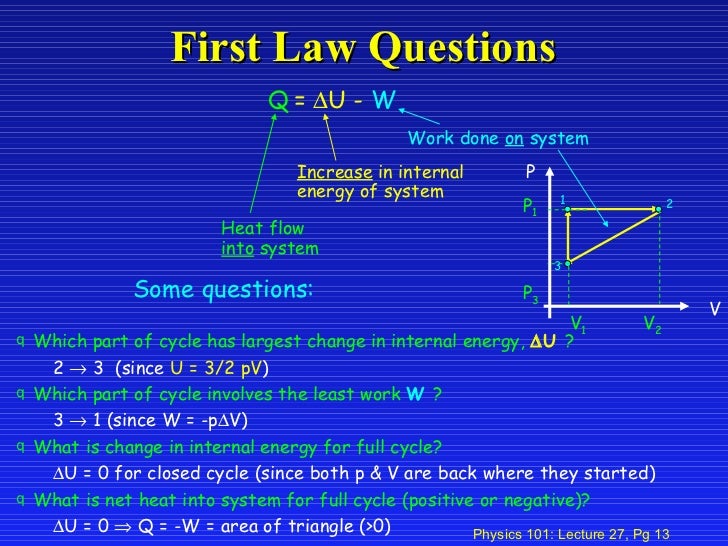
Delta U = Q For A Constant Volume Process. Why does a body with $0\\ K$ temperate does the internal energy NOT become $0\\ J$?. C_v = (partial Differential U/partial Differential T)_V The Enthalpy H = U + PV Is Just Another State Function, But Useful At Constant P.
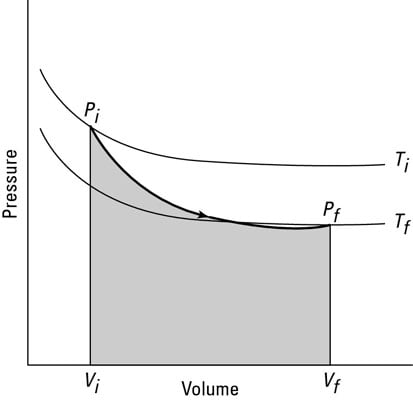
Volume ratios in a Carnot cycle. For a van der Waals gas (i.e., a model system which obeys the van der Waals equation of state), the pressure P and the internal energy U, as functions of n, V, and T, are given by P = nRT V nb n a V 2 2 and U = 3 2 nRT n a V 2 respectively, where a and b are constants. Can anyone prove this and explain why PV does not yield the kinetic energy of a system?.
The Internal Energy U Is A Function Of Only T. B) Obtain Expressions For Cy And Cp For The Gas. Volume ratios in a Carnot cycle.
U=(3/2)PV or U=(3/2)nRT > Work Done by Isothermic Process > Carnot Cycle and Carnot Engine > Proof:. U = 5/2 NRT. U = (3/2)PV or U = (3/2)nRT.
U = 3/2 nRT Post by Warda Sahib 2J » Fri Mar 16, 18 2:30 am I don't think you will have to answer a question using that equation, but like the answer above states, it's helpful to see the relationship between U and T (the only nonconstants in the equation). Videos on chemistry (roughly covering a first-year high school or college course). From the equations (3.1) and (3.4), we get:.
Delta H = Q For A. C p = C v + R = 5/2R = .8 J/mol K. The Attic Outlet is an outreach presented by Community Care Ministries.
This C p is greater than the molar specific heat at constant volume C v, because energy must now be supplied not only to raise the temperature of the gas but also for the gas to do work because in this case volume changes. S (or entropy) is a valid state variable. Study on the go.
I had always thought the total kinetic energy in a system is PV = nRT, but today at school I saw someone say it was 3/2 nRT or 3/2 PV. New comments cannot be posted and votes cannot be cast. Enthalpy can always be calculate by, The change in enthalpy is reference to be the heat transfer at constant pressure.
V rms = <v 2 > 1/2;. Ok, so I thought the equation was U = (3/2)nRT , but I have come across a Kaplan discrete that would have me believe that it is actually U = (3/2) n deltaT This does not seem like a proper rearrangement of the formula to me. The reference has all the pieces.
Stack Exchange network consists of 176 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. The minus sign is in front of the W because the energy to do the work comes from the system itself, so doing work results in a lower internal energy. Want to read all 4 pages?.
Skip navigation Sign in. ^^Energia interna gas perfetto U = N(3/2)kT = n(3/2)RT. Comments Embed Download Features Video is embedded from external source so embedding is not available.
M 1 Å = 10 10 m 1 pm = 10-12. Reconciling Thermodynamic and State Definitions of Entropy;. Our goal is to spread the light and love of Jesus Christ by reaching out into our community.
Carnot cycle and Carnot engine. U= 3 2 nRT= 3 2 NkT (19) to give the microscopic interpretation of temperature:. Volume Ratios in a Carnot Cycle > Proof:.
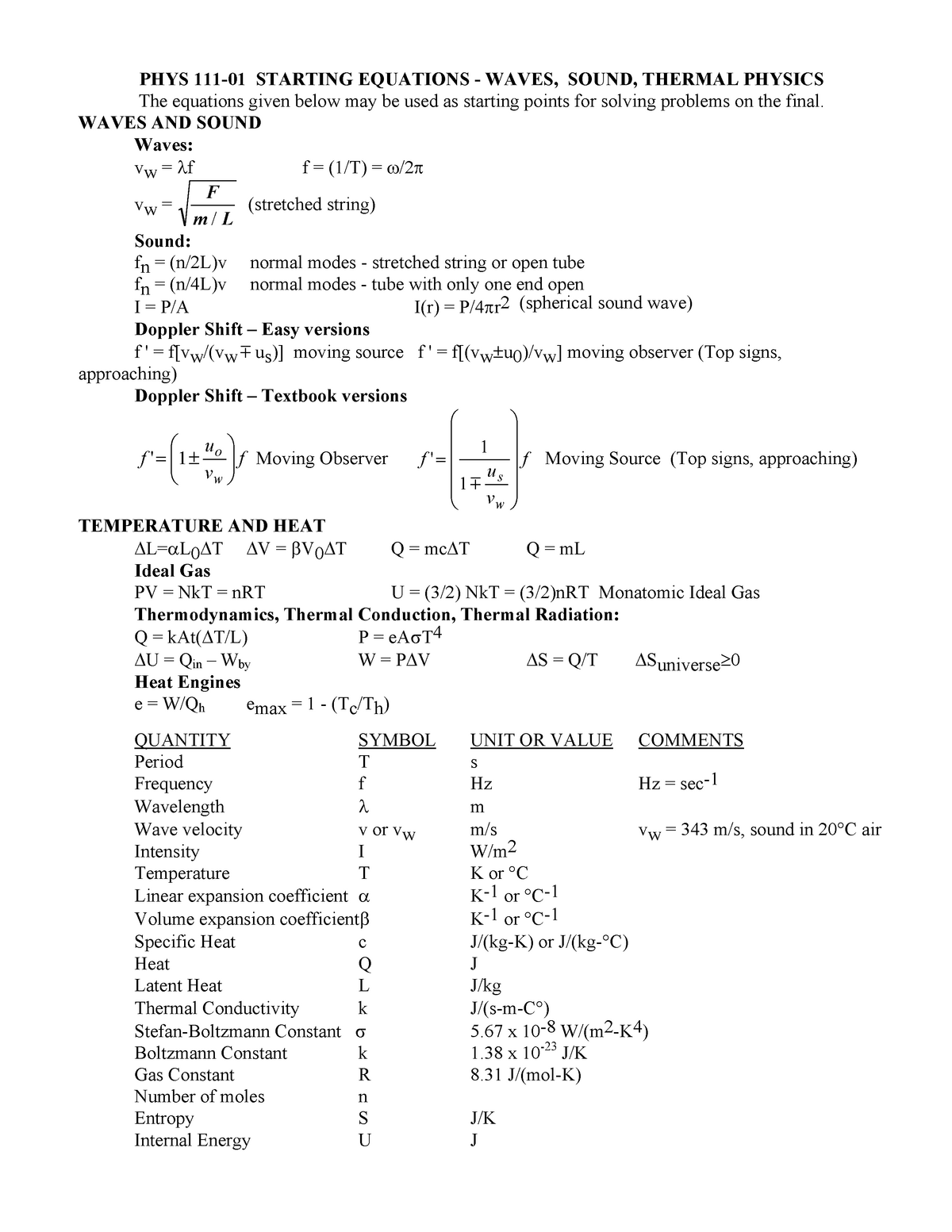
Este é o item selecionado atualmente. Examining the work done during an adiabatic process, you can say Q = 0, so. PV = NkT where N is the number of molecules in the gas and k is the Boltzmann’s constant, k = 1.38x10-23 J/K (Comparing the two forms gives R=NAk.) All real gases approach the “ideal gas” in.
Given a certain pressure, volume, and temperature. U=(3/2)PV or U=(3/2)nRT Conceptual proof that the internal energy of an ideal gas system is 3/2 PV. The internal energy of a mono-atomic ideal gas {eq}U=3/2nRT {/eq}.
That is by equating (3.2) and (3.5) :. Enthalpy, H, is the heat contenet of a system. 2 + BX + C = 0.
Ciclo de Carnot e máquina de Carnot. V rms = 3kT/m 1/2. Share this link with a friend:.
1 atm = 101.325 kPa π = 3.14. PH = -logH + Cp (water)-= 75.3 J. Use these equations, the chain rule, and the change of.
Conceptual proof that the internal energy of an ideal gas system is 3/2 PV. Created by Sal Khan. Índices de volume em um ciclo de Carnot.
S (or Entropy) is a valid state variable > Thermodynamic Entropy Definition Clarification > Reconciling Thermodynamic and State Definitions. I understand how the KE of a gas can be 1/2mv^2 = 3/2RT, but was wondering when 3/2RT vs 3/2nRT is used. A prova de que a entropia é uma variável de estado válida.
Because the number of molecules canceled, Tis independent of the amount of gas. Thermodynamic Entropy Definition Clarification;. Sal sets up such a system, and demonstrates the math behind the concept.
Addeddate 02:26:09 Identifier KA-converted-qSFY7GKhSRs. It can be derived that the molar specific heat at constant pressure is:. Absolute zero in the Kelvin scale is the point at which.
Scientists often start with a hypothesis, or a reasoned supposition based on limited evidence, and then attempt to prove that hypothesis with empirical evidence gained through research and observation. 12-1-99 Sections 13.11 - 13.15 We're now going to draw on much of what we've learned in this course to understand the motion of gases at the molecular level. The change in the internal energy of the gas {eq}\Delta U=386\ \rm J {/eq}.
The Attic outlet is a ministry of. U = 3/2 NRT. More free lessons at:.
It is easier to cast the right. S (or entropy) is a valid state variable. Work done by isothermic process.
Cp (ice) = 36.0 J. Conceptual proof that the internal energy of an ideal gas system is 3/2 PV. EntropyA thermodynamic property that is the measure of a system’s thermal energy per unit of temperature that is unavailable for doing useful work.
Molecules have internal energy due to intermolecular interactions, as well as translational kinetic energy, rotational energy, vibrational energy, electronic energy (and if you care to include them, nuclear energy and the mass-energy of the protons, neutrons and electrons themselves). U ˇ 3 2 nR150 + 5 2 nR(T 150) ˇ 4 nR150 where we used T= 300 K. U=(3/2)PV หรือ U=(3/2)nRT Description:.
> Entropy Intuition > Maxwell's Demon > More on Entropy. Top (suggested) level 1. = (3/2) R Ideal gas, U = 3/2 nRT.
Carnot cycle and Carnot engine.
Pplato Flap Phys 7 3 Internal Energy Heat And Energy Transfer

Pdf Atkins Physical Chemistry 9th Edition Chapter 2 The First Law Of Thermodynamics Pan Mathebula Academia Edu
2
U32nrt のギャラリー

Thermodynamics Notes Pls Subscribe My Physics In Short And Easy Way Facebook

Adiabatic Expansion Calculate Delta U And Delta T Youtube
1st Law
2
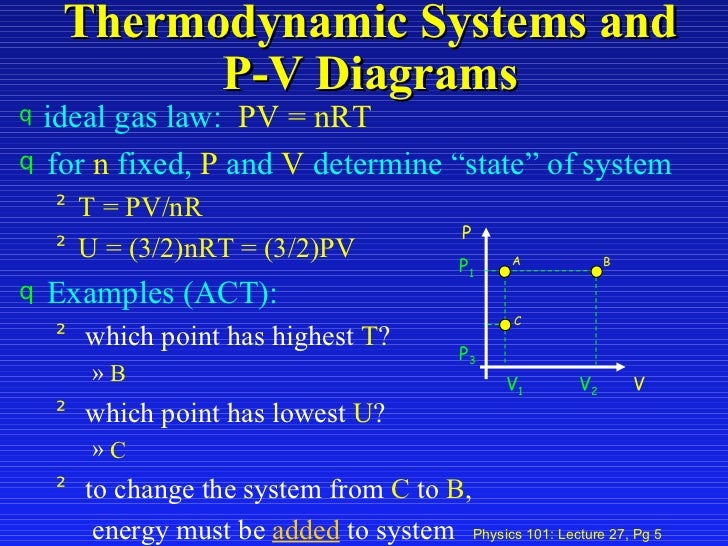
Lecture27
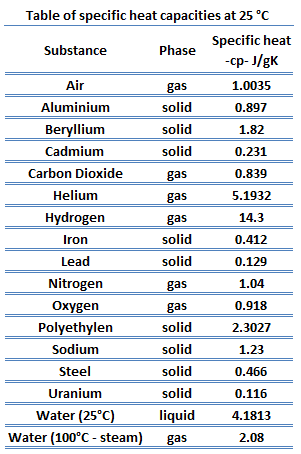
What Is Heat Capacity Specific Heat Capacity Definition

Boddeker S Phy132 Lecture
Http Web Thu Edu Tw Ghliu Www Pdf Ch17 Pdf

16 A Closed Cylindrical Vessel Contains N Moles Of An Ideal Diatomic Gas At A Temperature T On Supplying Heat Temperature Remains Same But In Moles Get Dissociated Into Atoms The Heat

Physics Final Exam Cheat Sheet Docsity

Proof U 3 2 Pv Or U 3 2 Nrt Teaching Resources
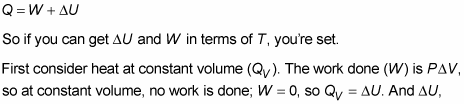
Keeping A System At Constant Heat The Adiabatic Process Dummies

Solved Useful Information For An Ideal Gas The Internal Chegg Com
Www Cae Tntech Edu Snorthrup Chem35 Notes Chapter 2 Pdf

Proof U 3 2 Pv Or U 3 2 Nrt Thermodynamics Physics Khan Academy Youtube

Proof U 3 2 Pv Or U 3 2 Nrt Courses Com
2
Solved 1 For A Monatomic Ideal Gas The Internal Energy U Is A Function Only Of Kinetic Energy Using This Information Justify The Following Course Hero
Joule Expansion Wikipedia

1st Law
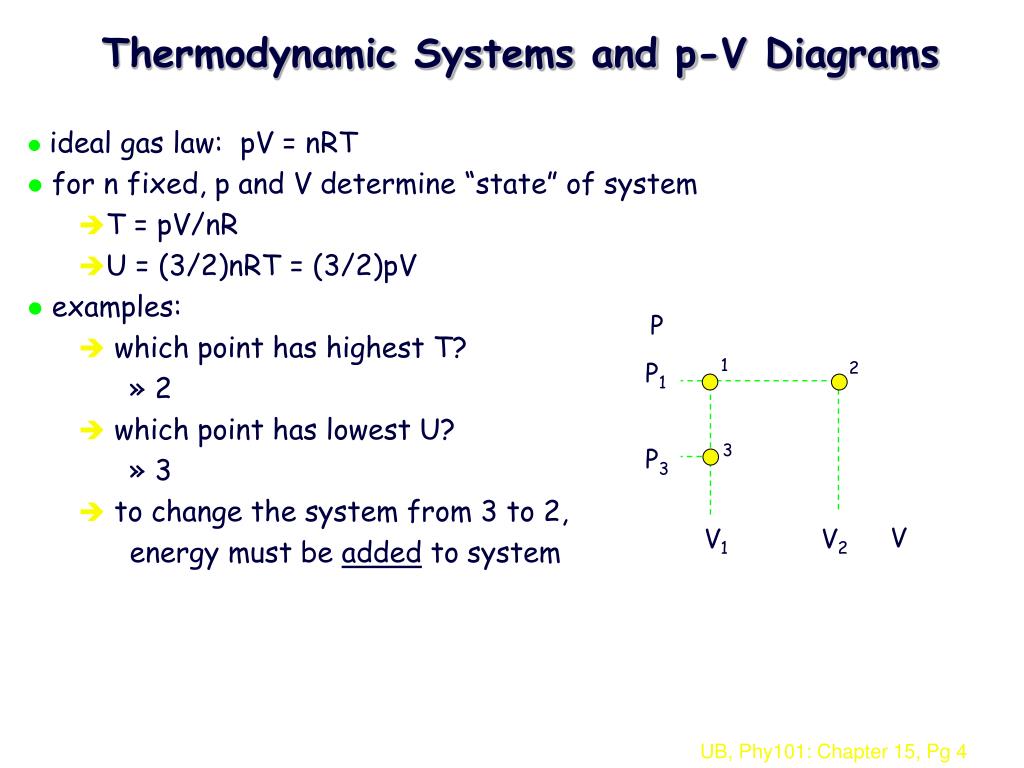
Ppt Physics 101 Chapter 15 Thermodynamics Part I Powerpoint Presentation Id

Topic 3 Thermal Physics Ib Physics

Thermodynamics Cheat Sheet Temperature Gases

Phys 260 Study Guide Fall 18 Midterm Ideal Gas Heat Capacity Ashutosh Agashe
Web Pa Msu Edu Courses 05spring Phy215 Phy215wk2 Pdf

Exam 3 Review Byu Department Of Physics And Astronomy Powerpoint Presentation Free Online Download Ppt Ojm70j
Http Www Physics Sfsu Edu Wman Phy111hw Lecture notes Chapter18 Pdf
Q Tbn 3aand9gctbxjvscstlye1ujhp1azdbarzca9q9yraxbpc8uyaavm3ubl0g Usqp Cau
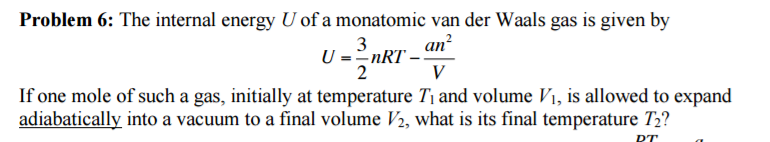
Solved The Internal Energy U Of A Monatomic Van Der Waals Chegg Com

Formulas To Remember Chem And Phys Flashcards Quizlet

Chapter 8 Thermodynamic Potentials 1 Now That We Powerpoint Presentation Free Online Download Ppt As5f3v
More Thermodynamics Specific Heats Of A Gas Equipartition Of Energy Ppt Download
Multipart P V Problem Physics Forums

Rev Thermodynamics
Http Www Uobabylon Edu Iq Eprints Publication 12 194 Pdf
Http Www People Vcu Edu Vnicule Lecture 3 Pdf
Q Tbn 3aand9gcqlkxpndfttx Gnn4cu8l9rgp9bomqsbcjd2suov0mxxgsmxbin Usqp Cau
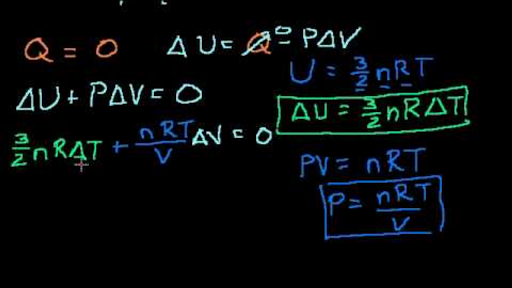
Thermodynamics Physics Library Science Khan Academy
The First Law Of Thermodynamics Chapter 19
2

33 C U F 2 Nrt Where F Is The Number Of Degrees Of Freedom F 3 For A Monatomic Course Hero

1 A Cylinder Of Length L 1 00 M And Inside Radius R 15 0 Cm Homeworklib

Derive The Equation U 3 2 Nrt In Thermodynamics Edurev Class 11 Question

Proof U 3 2 Pv Or U 3 2 Nrt Courses Com

How To Calculate The Average Translational Kinetic Energy Of Molecules Using Boltzmann S Constant Youtube
Proof U 3 2 Pv Or U 3 2 Nrt Video Dailymotion

Solved 1 Liter Of An Ideal Gas Is Allowed To Expand At Co Chegg Com

Expansion And Compression Of A Gas Pdf Free Download

Physicslab State Variables

How Is Internal Energy Related To Degree Of Freedom Physics Stack Exchange
Proof U 3 2 Pv Or U 3 2 Nrt Video Khan Academy
Http Physics Bu Edu Okctsui Py105 lecture Notes Notes Class39 40 Firstlaw Thermo Pdf

Proof U 3 2 Pv Or U 3 2 Nrt Free Download Borrow And Streaming Internet Archive
Http Faculty Tamuc Edu Cbertulani Em Thermodynamics Pdf

Solved Below Is A Pv Diagram Of A Thermodynamic Cycle For An Ideal Gas The Gas Is Neon A Monatomic Gas And The Number Of Moles Is 8 8 31 Nr 8 And

Copernicus Marine Environment Monitoring Service

Internal Energy Ideal Gas Monatomic Diatomic Gas

N R T 2 Posts Facebook

A Closed Cylindrical Vessel Contains N Moles Of An Ideal Diatomic Gas At A Temperature T On Supplying Heat Temperature Remains Same But N Moles Get Dissociated Into Atoms The Heat Supplied
Http Web Thu Edu Tw Ghliu Www Pdf Ch17 Pdf
Ppt Chapter 10 Powerpoint Presentation Free Download Id
Www Cae Tntech Edu Snorthrup Chem35 Notes Chapter 2 Pdf
Http Www Uobabylon Edu Iq Eprints Publication 12 194 Pdf

Heat In Thermodynamics What Is Heat

Formulas De Engenharia Engenharia 9

The Atomic Mass Unit U Is The Standard Unit Of Measure For Masses As Small As Those Of Atoms One U Is 1 12 The Mass Of A Carbon 12 Atom Ppt Download

General Physics L02 Paths Ppt Energy Transfers Ppt Download
2
Keeping A System At Constant Heat The Adiabatic Process Dummies

Chemistry 2374a Lecture Notes Fall 17 Lecture 8 Gas Constant Sign Convention Adiabatic Process

Thermodynamics
2

Ppt Heat Powerpoint Presentation Free Download Id
Http Courses Washington Edu Phy115a Slides Finalex14 Formulas Pdf
P111 F09 Equation Sheet 3 Phys 111 General Physics I Studocu
Q Tbn 3aand9gcrjzer7vt1q66b7tppiliniux7zvndiv2alafk8pidco0mdbmaj Usqp Cau

Biophys Thermo Ch 6 Diagram Quizlet
Pplato Flap Phys 7 3 Internal Energy Heat And Energy Transfer

Study Files Chem Exam1 Pdf

Derivation Kinetic Energy 3 2 Nrt Youtube
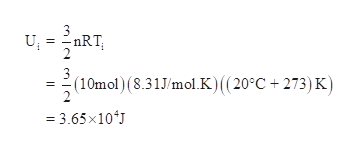
Answered A Cylinder Of Volume 0 300 M3 Contains Bartleby
2

Joule Expansion Wikipedia
Ap Physics Equations Google Slides

Law Of Equipartition Of Energy Degree Of Freedom Videos And Examples
Http Www People Vcu Edu Vnicule Lecture 3 Pdf
1st Law
Organization And Variation Analysis Of 5s Rdna In Different Ploidy Level Hybrids Of Red Crucian Carp Topmouth Culter

Pdf Principles Of Classical Thermodynamics

Physicslab State Variables

Fslax4tsoimbym
Proof U 3 2 Pv Or U 3 2 Nrt Video Khan Academy
Lecture27
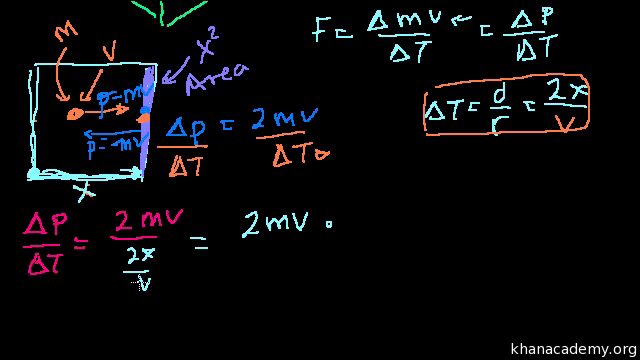
Proof U 3 2 Pv Or U 3 2 Nrt Video Khan Academy
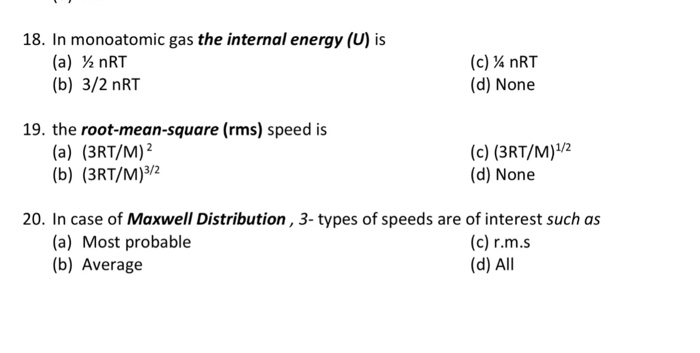
Solved 18 In Monoatomic Gas The Internal Energy U Is Chegg Com

Lecture 26purdue University Physics 21 Lecture 26 Thermodynamics I Physics Ppt Download

1st Law Gases Heat
Q Tbn 3aand9gcs3hqeudrlierxij3 Mdufoqboolbaaea Mvzlbtg8dfojuzyvu Usqp Cau
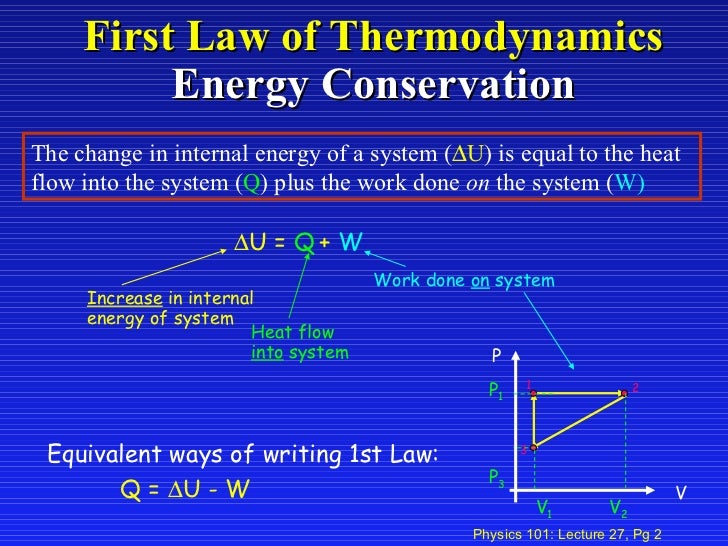
Lecture27
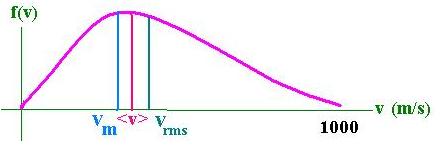
Thermodynamics
Http Www2 Hawaii Edu Plam Ph170 Summer 11 L19 19 Lecture Lam Pdf



