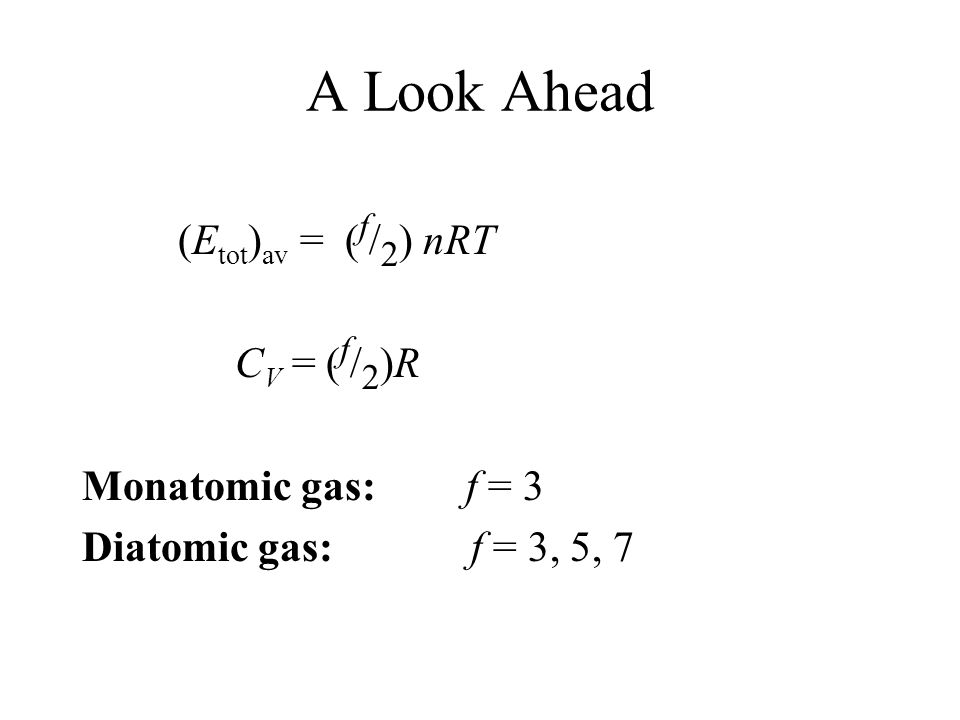
32nrt
This is the average force exerted by each single molecule.

32nrt. Created by Sal Khan. It gets a bit complicated to derive and you have to go through a long process. Since work argument above P(V2 - V1) = RT is simple and holds for all gases, This suggests KE > (3/2)RT for diatomics, This would make Cp/Cv < 1.67 Equipartition Theorem:.
I know that subsequently, it will be 2/3KE= nrt and i will get KE=3/2 nRT i just don't KNOW WHERE. Kav= (3/2)kT Here we have a fundamental connection between temperature and the average translational kinetic energy of the atoms - they are directly proportional to one another. Consider A Heat Engine That Operates Between A Hot Reservoir At 150 DegreeC And A Cold Reservoir At 30 DegreeC.
Temperature is a measure of the average kinetic energy of the atoms. (3/2)nRT is the translational kinetic energy, and since almost all atoms are in the ground electronic state at low temperature, it is a good expression for internal energy as long as the temperature is low enough that essentially all atoms are in the electronic ground state. When a gas is stored under pressure in a closed vessel is discharged to the atmosphere through an orifice, the gas velocity through that orifice may be choked or non-choked.
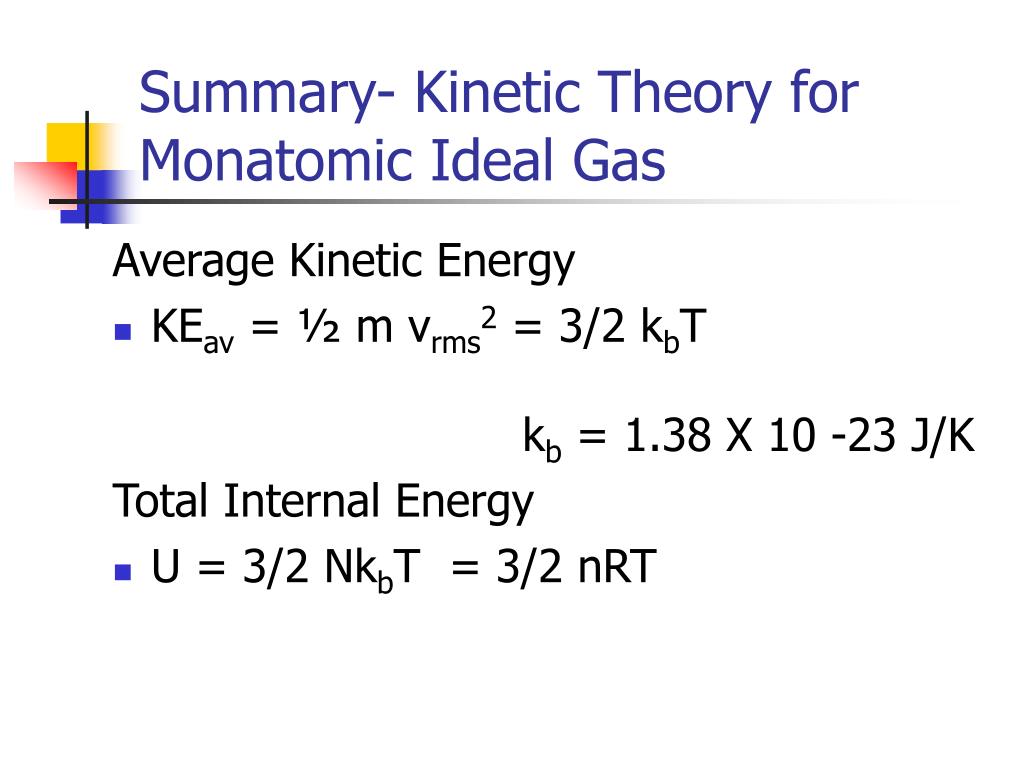
This is primarily a memory refresher. Helium, neon, argon) with the absolute temperature. It’s also used to calculate the internal energy of a monatomic ideal gas.
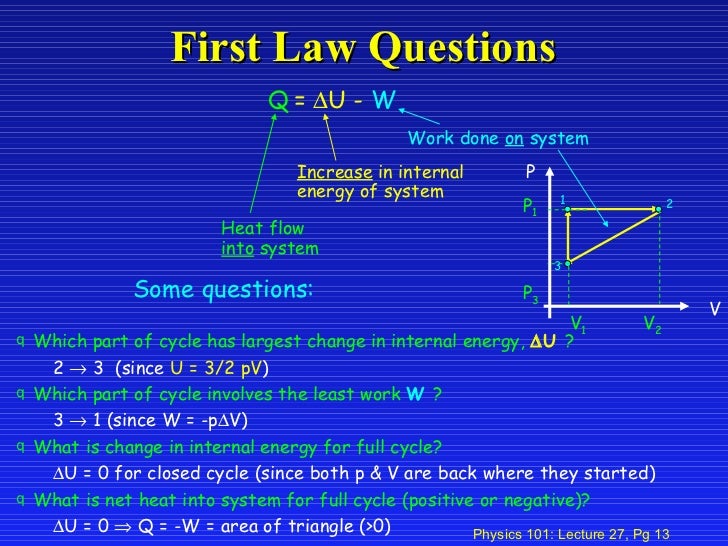
• U is called a “state variable” because once the state of the system is specified, such as the number of moles and the temperature or pressure and volume, U has an unique value, that is U is function U(n,T) or U(P,V). Gas Discharge Rate Atmosphere From a Pressure Vessel. It can be derived that the molar specific heat at constant pressure is:.
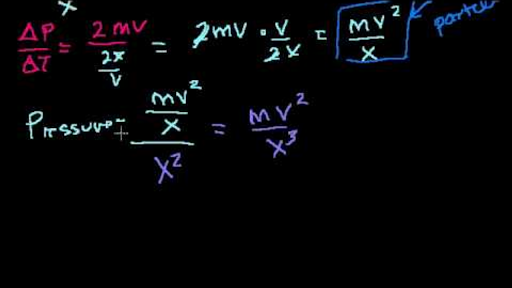
I'm taking a test tomorrow. Key Takeaways Key Points. PV = 1/3 mN << upsilon^2 >> where m is the mass in "kg" and N is the number of molecules.
17.2 The molecular partition function • The energy of a molecule is the sum of contributions from its different modes of motion:. Derive equation for Ek of a molecule. But we have N/3 molecules hitting this wall.
Equation of Ek of a molecule. PAd = some tiny work done by n moles of the gas, d is a tiny distance moved v = Ad = tiny volume expanded or contracted N/n = ratio between total moles. This C p is greater than the molar specific heat at constant volume C v, because energy must now be supplied not only to raise the temperature of the gas but also for the gas to do work because in this case volume changes.
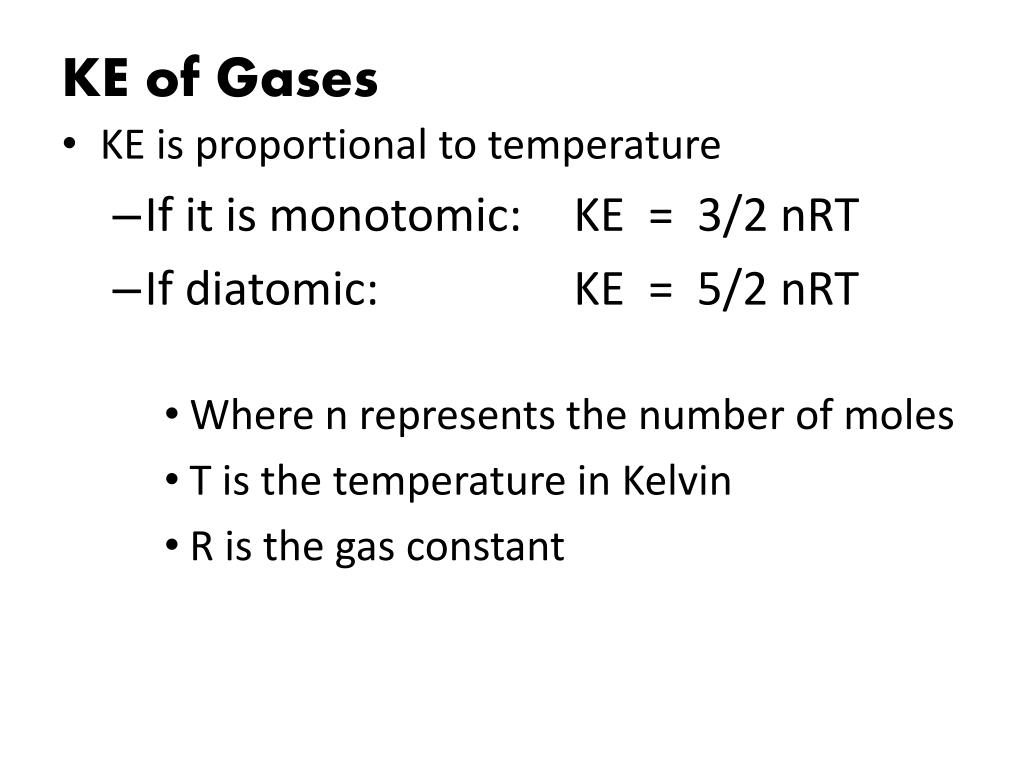
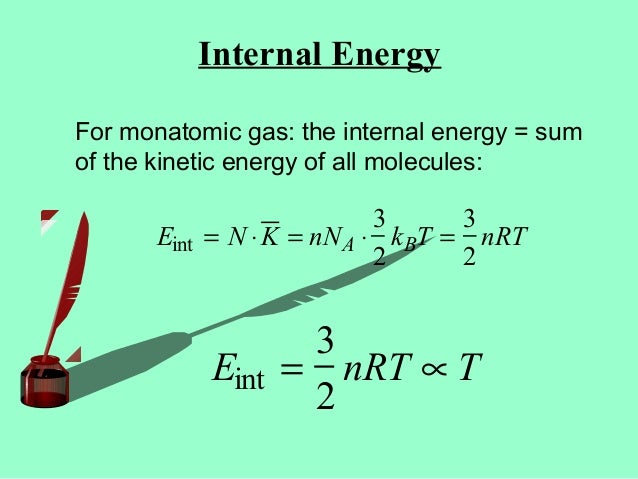
Ekin = 3/2nRT, internal energy (change in U), translational kinetic energy;. All gases in the problem are considered as ideal gases, the internal energy of ideal gas is given by U(T,V,n)=3/2nRT. Ok Pressure * Volume = Numbe rof moles * molar gas constant * Temperature I have got it down to 1/2m*meansquaredspeed = (3/2nRT)/Number of particles I know i am suppose to use avogadro equation and then use the Boltzman constant but the numbers are not popping out.
This is where the equipartition of energy idea comes in – any other contribution to the energy must also contribute (1/2)nRT. Kinetic Molecular Theory of Gases Basic Concept Kinetic Molecular Theory of Gases. Kinetic-Molecular Theory is to model the properties of an ideal gas.
It's the kinetic energy of the molecules. Can someone just do the maths for me please to get 1/2m*meanssquaredspeed = 3/2kT Thank you!. Eint = 3/2 NkT = 3/2 nRT where n is the number of moles.
What is the average kinetic energy and velocity of nitrogen gas molecules at 273 K and at 546 K?. If the length of the cube, mass of the molecule and velocity are l, m and v respectively, Momentum in the x-direction = mU x Momentum in the -x-direction = -mU x Change in momentum = 2mU x Total time taken - from one end to the other and vice versa - = 2l / U x Rate of change in momentum = 2mu/(2l / U x) = mU x 2 /l According to Newton's Second Law, the rate of change of momentum is the force. I keep remembering 8.354, but i'm not sure that's correct.
A 2.10 mol sample of oxygen gas is confined to a 5.10 L vessel at a pressure of 7.99 atm. KE1/KE2=1/1(at equal number of moles and constant temperature. Pls answer only if you are sure.
I think that would be one of the only instances to use it. This leads to the expression where N is the number of molecules, n the number of moles, R the gas constant, and k the. KE = 3/2 RT for ideal gases.
When to use E=3/2nRT?. The two equations agree when the average translational kinetic energy of the molecules is:. Find the average translational kinetic energy of an oxygen molecule under these conditions in (j/mol).
For any feedback or comments, contact a fellow IB alumni:. Demonstração conceitual de que a energia interna de um sistema de gás ideal é de 3/2 PV. When should the equation be used because I have yet to see where the formula applies?.
However, from my notes, "KE=3/2nRT=3/2kT" is applicable to all kind of gas" made me doubtful. For diatomics and polyatomics find Cp/Cv < 1.67!. C p = C v + R = 5/2R = .8 J/mol K.
Of mass reference frame and it is U=3/2nRT=3/2PV. This of course assumes the molecule is not subject to an external potential. 4) Pressure is due to collisions of gas particles with container walls.
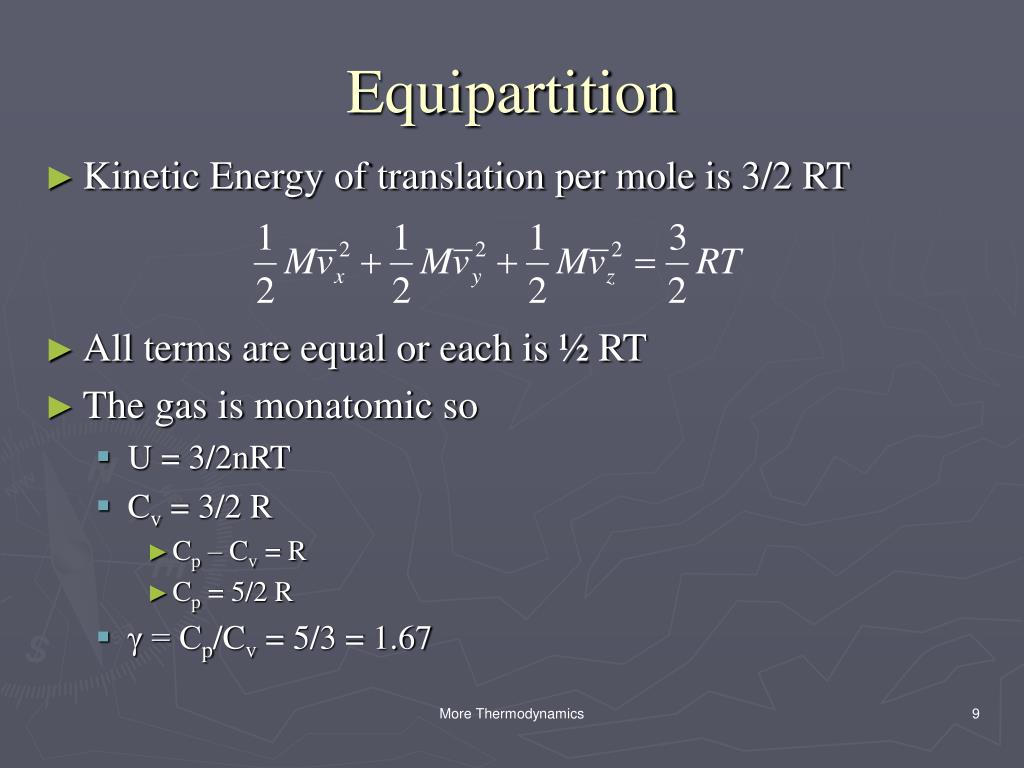
Each direction (x, y, and z) contributes (1/2)nRT to the internal energy. Conceptual proof that the internal energy of an ideal gas system is 3/2 PV. PV=NkT PV=1/3Nmc2 equate equations NkT=1/3mnc2 mc2=3kT 1/2mc2=3/2kT Ek=3/2kT.
If it is your first exposure to this material, then it may be a little too quick. The equipartition principle gives 3/2nRT for the internal energy of a perfect monatomic gas. 14), from a derivation based on the kinetic theory of gases, an ideal gas has:.
Can anyone me what the number for r always is?. Below is the question Mere questions pr muze galat Boland vale donkey hoge sale If A and B are perpendicular to each other, prove that IA +B I = √ + B2 Do you sex with me zoom meeting pr I'd de do now iam naked nhi tum de do kse doge yrr tumne I'd galat Diya hai Sahi de do instra nhi use karti In the circuit, the potential difference between A and B is Unit. What are the values of {eq}(dU/dV)_{T} {/eq} and {eq}(dH/dV)_{T} {/eq} for a perfect gas?.
May 10, 18 #2 The 3/2 term comes from using the kinetic theory of gases to determine the relationship between the average translational kinetic energy of a monatomic ideal gas (e.g. From Levine's "Physical Chemistry" (pg. Can anyone prove this and explain why PV does not yield the kinetic energy of a system?.
Or I have misunderstanding to the notes?. Learn what the first law of thermodynamics is and how to use it. I need to use the kinetic energy formula, k=3/2nrt.
I know that t is the temperature, and n is the number of moles. This implies that there are 2 axes of rotation. I had always thought the total kinetic energy in a system is PV = nRT, but today at school I saw someone say it was 3/2 nRT or 3/2 PV.
Why the formula need not consider degree of freedom?. This is a very general law which. The kinetic molecular theory states that an ideal gas' KE is only affected by temperature.
The Working Fluid Is A Non-ideal Gas For Which The Physical Equation Of State Is P = NRT / V - An^2 / V^2 And The Energy Equation Of State Is U = 3/2nRT - An^2 / V. The ideal gas law assumes that gases are composed of point masses that interact via completely elastic collisions. Hi i am working on trying to derive KE (or Etrans)=3/2nRT so i know these steps that ___________ pV= nRT and pV= 1/3 NmV2 and combining them, we get nRT= 1/3Nmv2 also, we know that for translational motion, KE= 1/2Nmv2 ______ i don't know how 2/3 (1 /2 Nmv2) = nRT where did that 2/3 come from??!!.
When to use E=3/2nRT?. 3/2kT is the avg ke of each gas molecule. Molecules are, in a great numbers, everywhere and there is no vacuum at 100%.
Dec 9, 16 #4 Hiranya Pasan. Assume one-third of the molecules move along the x-axis at constant speed v. With that said, I'm still not understanding how/why mass does or does not play a role.
1 visitor has checked in at Ek=3/2PV=3/2nRT=3/2NkBT. And it's really everything thrown in there. Therefore one axis contribute 1/2KT in rotational K.E , therefore rotational K.E of a diatomic gas is KT.
I've already told you multiple times that big, uppercase U is the internal energy of a system. Post by Chem_Mod » Fri Aug 19, 11 12:38 am. Translation, vibration, rotation, electronic) and the number of coordinates necessary to describe each type of motion.
E int = 3/2nRT J Molar specific heat at constant volume Q = nC V ΔT J C V = 12.5 J/mol*K, monatomic, .8 J/mol*K, diatomic, 24.9 J/mol*K, polyatomic Molar specific heat at constant pressure Q = nC p ΔT J C p = C V + R Adiabatic expansion pV γ = constant TV γ-1 = constant Change in entropy ΔS = ∫dQ/T J/K ΔS = nR*ln(V f /V i) + nC V *ln. My thinking was this:. Root mean square speed.
Ek is independent of. 3) Gas particles neither attract nor repel each other. 5) The average kinetic energy of a gas sample is proportional to the Kelvin Temperature.
The simple answer is that the 2 comes from the equation for kinetic energy, 1/2mv^2 which you should have had in physical science. Such that cv = 3/2R degrees of freedom different types of motions available to molecules (e.g. 3/2 nRT is the average KE per mole of gas.
Pressure Vessel Engineering and Design Fluids Engineering. Lavelle has also used it to show that deltaU is 0 during isothermic processes, but I don't think we will use the equation too much. KE = 3/2 RT where R = 8.3145 J/mol.
Average kinetic energy is prop. But, i can't remember what the number is. It was first stated by Émile Clapeyron in 14 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law.
I know that r is the constant. Depends on the units you want. Watch the next lesson:.
Kinetic theory of gases is a branch of Statistical Mechanics.It allows us to understand the behavior of molecules in terms of macroscopic quantities by using averages. The change in the internal energy of the gas {eq}\Delta U=386\ \rm J {/eq}. The reference has all the pieces.
Conceptual proof that the internal energy of an ideal gas system is 3/2 PV. But what is temperature, according to the theory?. Hot Products Antigens & Antibodies for Diagnostic Kit DevelopmentNew SARS-CoV-2 Related ProductsNew CD19 - a validated therapeutic target for B-cell malignanciesNew CD3 Protein - A hot target for bispecific antibodyHot CD24 & Siglec-10 Proteins - Hot Targets for Tumor ImmunotherapyNew CD73 - A novel target for immunotherapyNew Siglec-15 - Validated by Cell-based Assay BLI/ SPR/ ELISANew CD.
Kinetic Temperature The expression for gas pressure developed from kinetic theory relates pressure and volume to the average molecular kinetic energy.Comparison with the ideal gas law leads to an expression for temperature sometimes referred to as the kinetic temperature. Provide algebraic expression for all intermediate answers, you may provide a numerical value only for the final answer, i.e for the enthalpy ?(f) H of formation. This video is a very quick, all math, derivation of K.E.= (3/2)nRT= (3/2)PV.
Foursquare uses cookies to provide you with an optimal experience, to personalize ads that you may see, and to help advertisers measure the results of their ad campaigns. (17.10) • T denotes translation, R rotation, V vibration, and E the. I'm guessing your E_k is the average kinetic energy per mol.
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. Or for one mole of diatom. For each molecule in diatomic molecules there are axes of rotation.
Thu Aug 04, 11 8:53 pm Has upvoted:.
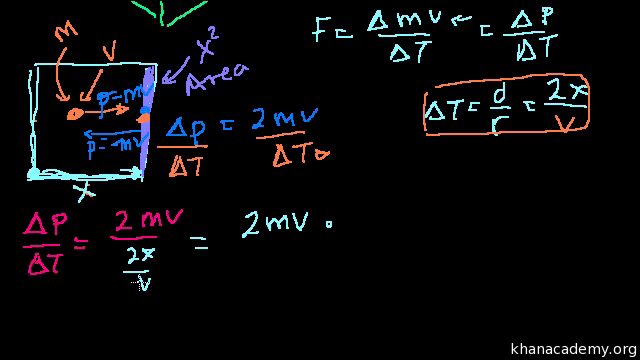
Proof U 3 2 Pv Or U 3 2 Nrt Video Khan Academy
Http Www Physics Sfsu Edu Wman Phy111hw Lecture notes Chapter18 Pdf
Ppt The Gas Laws Powerpoint Presentation Free Download Id
32nrt のギャラリー
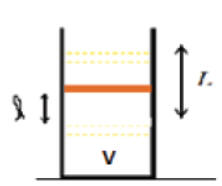
Lecture27
2

1st Law
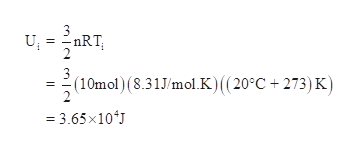
Answered A Cylinder Of Volume 0 300 M3 Contains Bartleby

Docplayer Net Docs Images 41 Images Pa
2
1st Law
Q Tbn 3aand9gctkiyhr6lnvhsumziux66dpu3yejtomv6tkiqooin0zxdhvfwxj Usqp Cau

Lecture27
Q Tbn 3aand9gcqlkxpndfttx Gnn4cu8l9rgp9bomqsbcjd2suov0mxxgsmxbin Usqp Cau

Teoria Cinetica Dos Gases
2

Derivation Kinetic Energy 3 2 Nrt Youtube

2 The Total Kinetic Energy Of 1 Mole Of N2 At 27a C Will B Scholr
Proof U 3 2 Pv Or U 3 2 Nrt Video Dailymotion
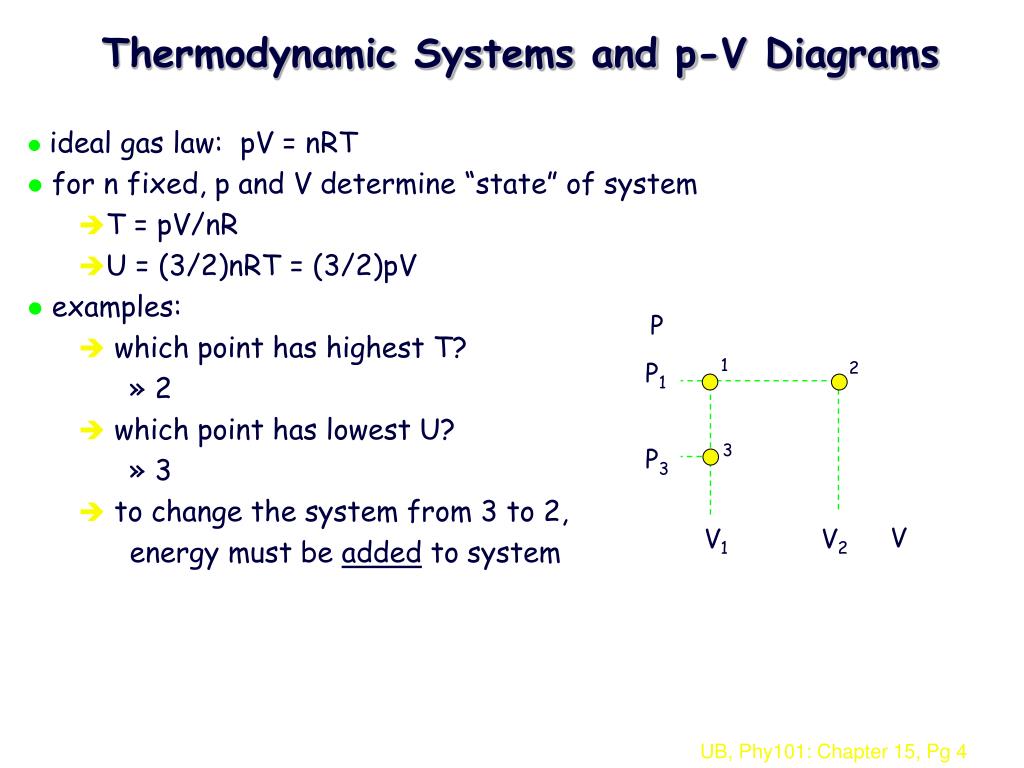
Ppt Physics 101 Chapter 15 Thermodynamics Part I Powerpoint Presentation Id
Lecture27
Q Tbn 3aand9gcrjzer7vt1q66b7tppiliniux7zvndiv2alafk8pidco0mdbmaj Usqp Cau
Graddiv Ucsc Edu Training Pdfs Garp Manual Mod 6 Pdf

Solved Useful Information For An Ideal Gas The Internal Chegg Com

Lecture 26purdue University Physics 21 Lecture 26 Thermodynamics I Physics Ppt Download
When An Ideal Monoatomic Gas Is Heated At Constant Pressure Sarthaks Econnect Largest Online Education Community
2

Proof U 3 2 Pv Or U 3 2 Nrt Free Download Borrow And Streaming Internet Archive

Proof U 3 2 Pv Or U 3 2 Nrt Thermodynamics Physics Khan Academy Youtube

The Ratio Of A Specific Heats Of A Gas Is 9 7 Then The Number Of Degrees Of Freedom Of The Gas Molecules For Translation Motion Is

A Closed Cylindrical Vessel Contains N Moles Of An Ideal Diatomic Gas At A Temperature T On Supplying Heat Temperature Remains Same But N Moles Get Dissociated Into Atoms The Heat Supplied
2
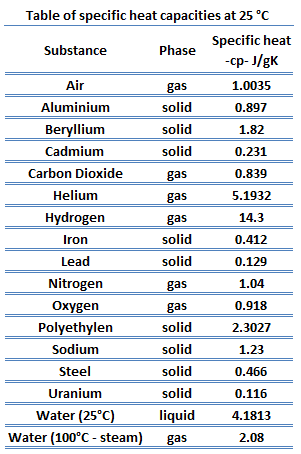
What Is Heat Capacity Specific Heat Capacity Definition
Find The Change In The Kinetic Energy Of An Ideal Gas Page 2 Physics Forums
Http Web Thu Edu Tw Ghliu Www Pdf Ch17 Pdf

General Physics L02 Paths Ppt Energy Transfers Ppt Download

Prove That The K E Per Unit Volume Of An Ideal Gas Is 3 2 P

U 3 2 Nrt Proof Ke 3 2nrt Ke Equals 3 Divide By 2 Nrt For A Monoatomic Ideal Gas Kisembo Youtube

Thermodynamics

Neet Exam How Is It 3 2 Nrt Chemistry Meritnation Com
Joule Expansion Wikipedia

Neet Exam How Is It 3 2 Nrt Chemistry Meritnation Com

How Is Internal Energy Related To Degree Of Freedom Physics Stack Exchange
Ppt Chapter 10 Powerpoint Presentation Free Download Id

Chem 123 Study Guide For Physical Chem Chem 123 Ubc Studocu

Preschool Language And Phonological Proficiencies In Predicting Stuttering Recovery Or Persistence Semantic Scholar

Degree Of Freedom Physics For Scientist And Engineers Past Paper Docsity
Q Tbn 3aand9gctzyc1hwwy5jzt 2o1ldoyn Ie9uvmi Q8v6zawcd8cusgizecu Usqp Cau

Exam 3 Review Byu Department Of Physics And Astronomy Powerpoint Presentation Free Online Download Ppt Ojm70j

Ned Nikolov Ph D In Order To Understand How Pressure Affects The Kinetic Energy And Temperature One Must Understand The Fundamental Relationship Between Force Which Pressure Is Energy And Temperature Energy
Www Cae Tntech Edu Snorthrup Chem35 Notes Chapter 2 Pdf

Phys 260 Study Guide Fall 18 Midterm Ideal Gas Heat Capacity Ashutosh Agashe
Proof U 3 2 Pv Or U 3 2 Nrt Video Khan Academy
Kinetic Theory Of Gases
2

Internal Energy Thermodynamics For Iit Jee Unacademy

What Is Average Kinetic Energy Of 1 Mole Of So2 At 300k Brainly In

Physics I Gases Flashcards Quizlet
Ppt More Thermodynamics Powerpoint Presentation Free Download Id

What Is Average Kinetic Energy Of 1 Mole Of So At 300 K 1 4578 J Mol 2 3134 J Mol 4 4173 J Mol 3 Brainly In

Thermodynamics Notes Pls Subscribe My Physics In Short And Easy Way Facebook
Http Web Thu Edu Tw Ghliu Www Pdf Ch17 Pdf
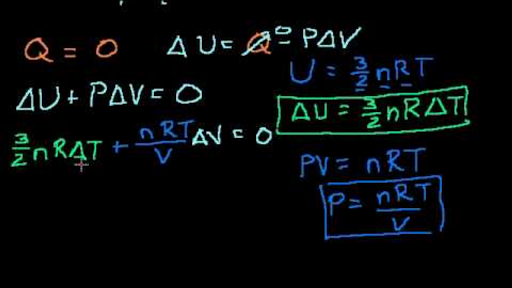
Thermodynamics Physics Library Science Khan Academy

16 A Closed Cylindrical Vessel Contains N Moles Of An Ideal Diatomic Gas At A Temperature T On Supplying Heat Temperature Remains Same But In Moles Get Dissociated Into Atoms The Heat

Gases And Heat Chapter Ppt Video Online Download

オリジナル32nrt
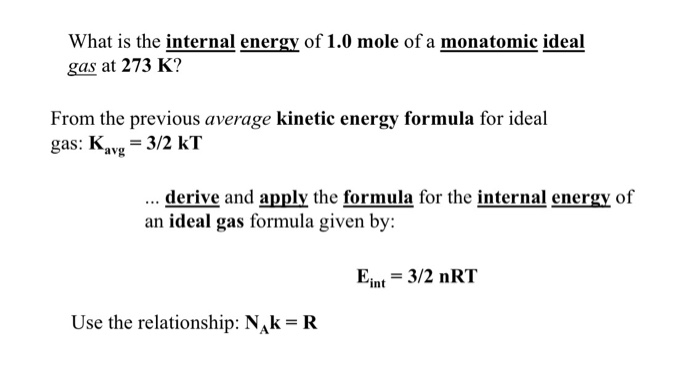
Solved What Is The Internal Energy Of 1 0 Mole Of A Monat Chegg Com
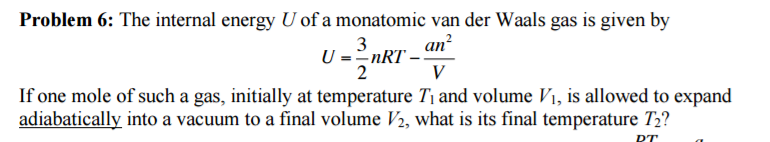
Solved The Internal Energy U Of A Monatomic Van Der Waals Chegg Com
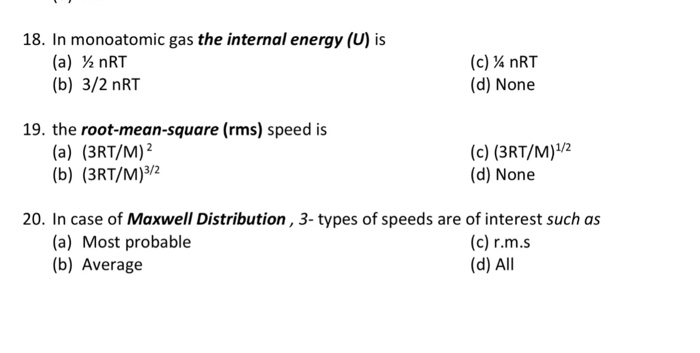
Solved 18 In Monoatomic Gas The Internal Energy U Is Chegg Com
Http Www2 Hawaii Edu Plam Ph170 Summer 11 L19 19 Lecture Lam Pdf

Degree Of Freedom Physics For Scientist And Engineers Past Paper Docsity
2

U 3 2 Pv أو U 3 2 Nrt اثبات Youtube

Homework 5 Homework 5 If Two Objects Are In Thermodynamic Equilibrium Cant Be At Diff Temp Thermometer Substance Undergoes Some Change When Heated Or Course Hero
Thermal Properties Of Matter Ppt Video Online Download

Proof U 3 2 Pv Or U 3 2 Nrt Courses Com

Internal Energy Of An Ideal Gas Molar Heat Capacity Of Monatomic Diatomic Gases Gamma Ratio Youtube

Univariate Factors Related To Cessation At 38 Weeks N 67 Download Table

Manual E Solucoes Halliday 7ed Fisica Iii 36

Physicslab State Variables

44 What Is The Ratio Of The Average Molecular Energy Of Ufg To That Of H Both At 300 K 1 1 1 2 349 2 3 2 349 4 None Of These

1 A Cylinder Of Length L 1 00 M And Inside Radius R 15 0 Cm Homeworklib
Plos One Organization And Variation Analysis Of 5s Rdna In Different Ploidy Level Hybrids Of Red Crucian Carp Topmouth Culter
2

Physics I Gases Flashcards Quizlet

Boddeker S Phy132 Lecture
Ap Physics Equations Google Slides

The Temperature Of N Moles Of An Ideal Gas Is Increased From T To

Internal Energy Ideal Gas Monatomic Diatomic Gas

Solved Part 2 Gas Calculations Ke 3 2nrt R 0 006 L A Chegg Com
State And Equal Intrinsic Energy Curved Surfaces Of Ideal Gas

Consider An Equimolar Mixture Of Monoatomic Gas And Diatomic Gas The Heat Required To Increase The
2
What Would Be The Average Kinetic Energy Of A Hydrogen Molecule At 300k Quora
Pplato Flap Phys 7 3 Internal Energy Heat And Energy Transfer
View Question

Rev Thermodynamics
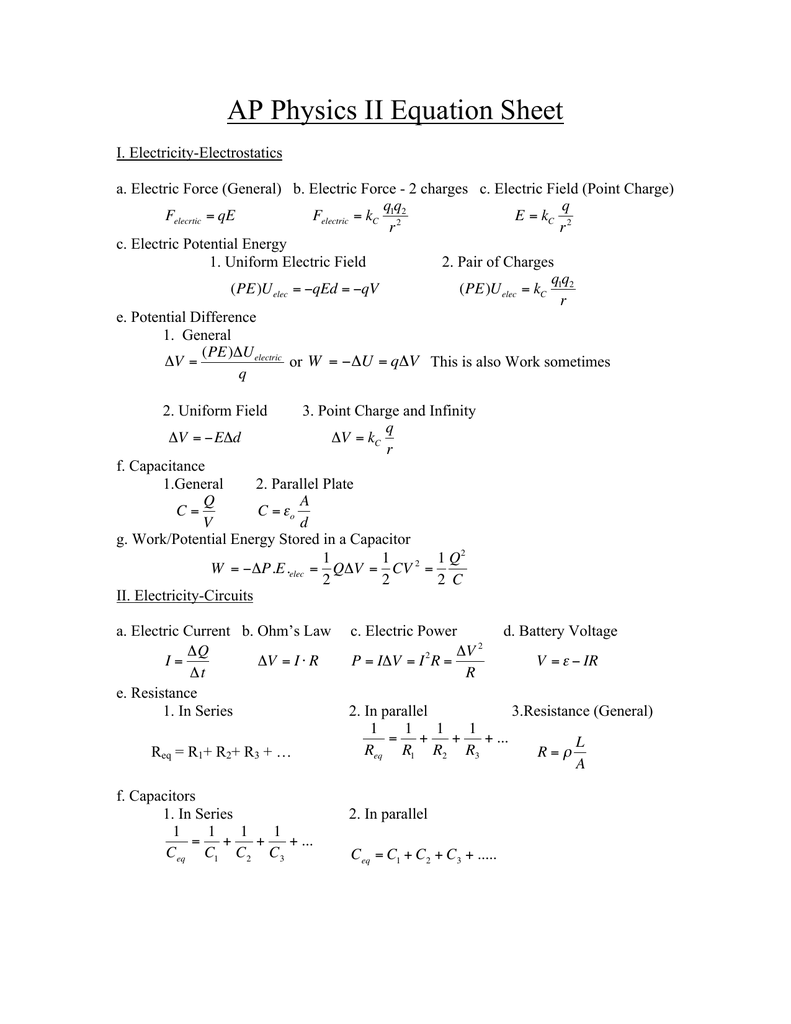
Apphysicsii Equation Review Summer Manualzz
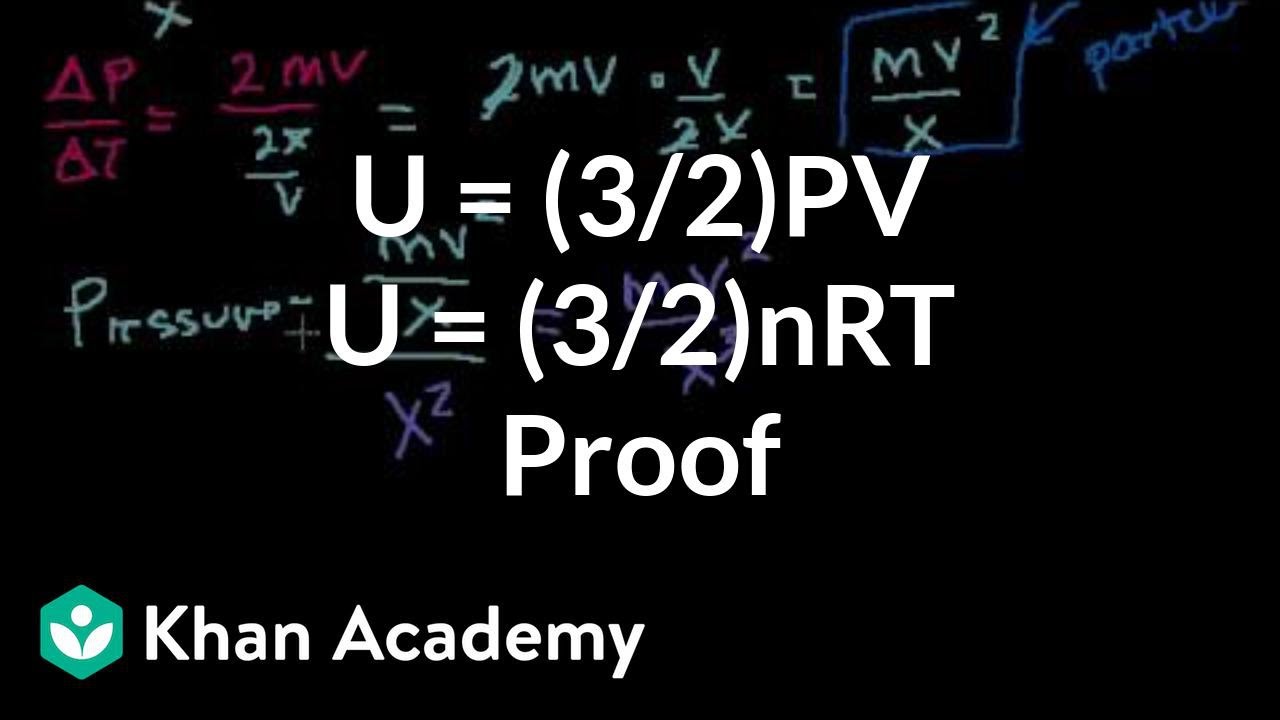
Proof U 3 2 Pv Or U 3 2 Nrt Video Khan Academy
2

Specific Heat Of Gases N Moles Of An Ideal Diatomic Gas Are In A Cylinder At Temperature T Suppose On Supplying Heat To The Gas Its Temperature Remain Constant But N Moles

Week 8 Quiz Docx Week 8 Quiz Grade 95 Ph2 Question 1 15 Out Of 1 What Happens To The Internal Energy Of Water Vapor In The Air That Condenses On Course Hero

Physics Thermodynamics 1 Nrt In 2 2 Nrt In 2 2 3 41 Boa Cold Storage Ice Melts At The Rate Of 2 Kg N When The Extern Kunduz

Thermodynamics Cheat Sheet Temperature Gases
2



